“Understanding Acid Rain: Causes, Effects, and Sustainable Solutions”
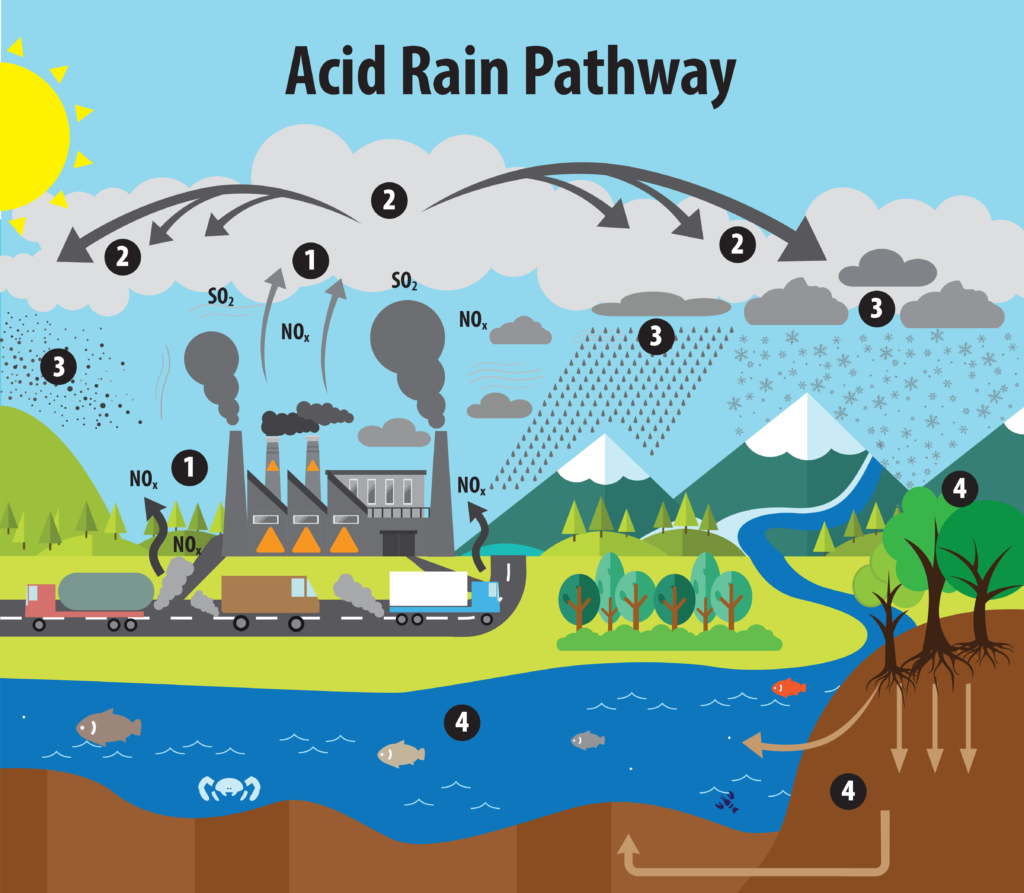
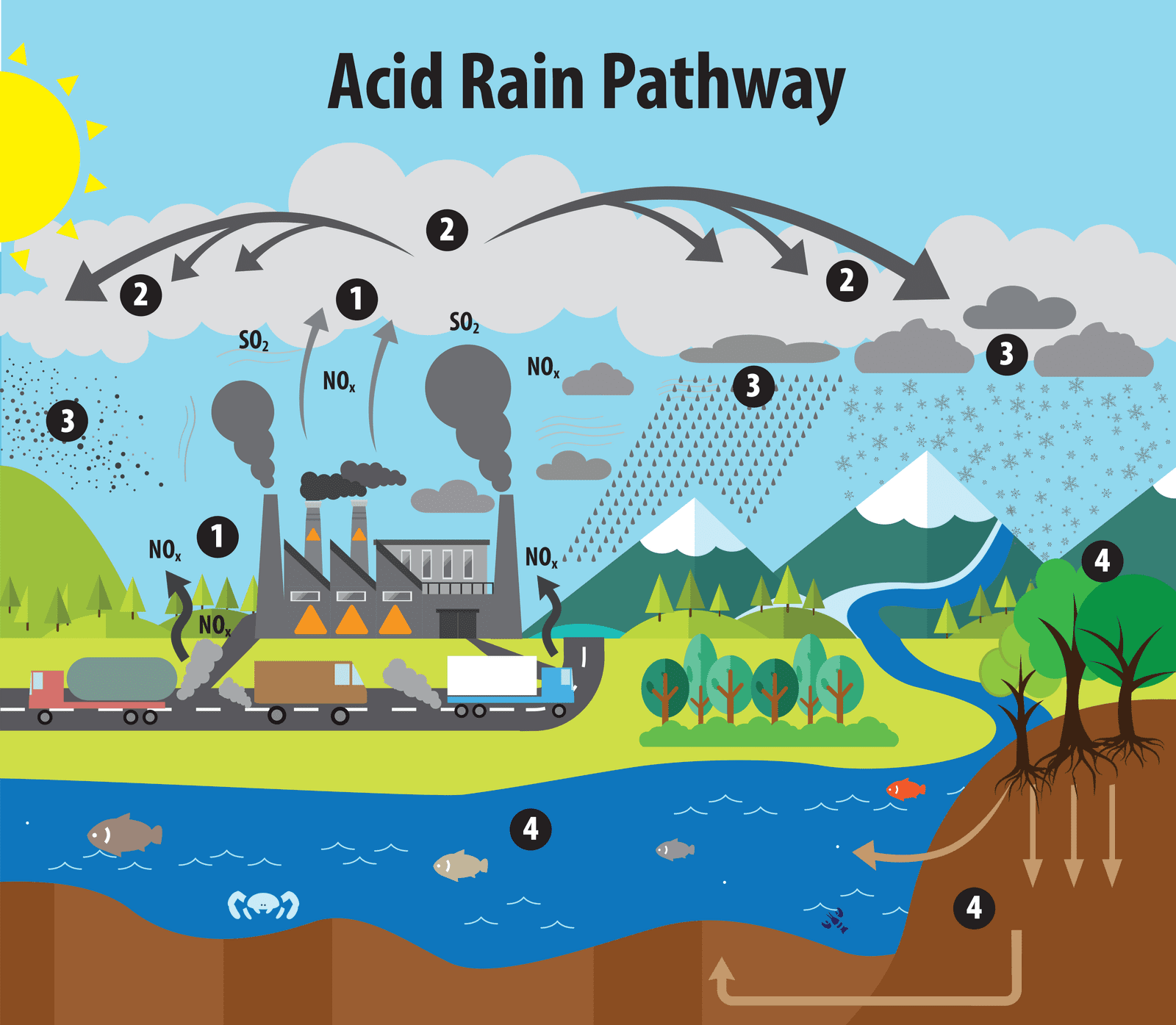
Acid rain is a form of environmental pollution characterized by rainwater, snow, or any form of precipitation that becomes acidic due to the presence of pollutants in the atmosphere. The primary cause of acid rain is the emission of sulfur dioxide (SO2) and nitrogen oxides (NOx) into the air, which react with water, oxygen, and other chemicals to form sulfuric acid (H2SO4) and nitric acid (HNO3). These acids are then carried by the wind and deposited on the Earth’s surface when it rains or snows.
“Effective Strategies to Control the Greenhouse Effect: Mitigating Climate Change and its Impact”
Main causes of Acid Rain
The causes of acid rain are primarily related to the emission of certain pollutants into the atmosphere. These pollutants undergo chemical reactions that result in the formation of acidic compounds. The main causes of acid rain include:
- Fossil Fuel Combustion: The burning of fossil fuels, such as coal, oil, and natural gas, in power plants, industries, and vehicles releases significant amounts of sulfur dioxide (SO2) and nitrogen oxides (NOx) into the air. These gases react with atmospheric moisture to form sulfuric acid (H2SO4) and nitric acid (HNO3), respectively.
- Industrial Emissions: Various industrial processes, including manufacturing, metal smelting, and chemical production, emit sulfur dioxide and nitrogen oxides, contributing to acid rain formation.
- Transportation Emissions: Motor vehicles, especially those using diesel engines, release nitrogen oxides, which are major contributors to acid rain.
- Residential Activities: Burning of fossil fuels for heating and cooking in households also contributes to the release of sulfur dioxide and nitrogen oxides.
- Natural Sources: While human activities are the primary cause of acid rain, natural sources can also release sulfur dioxide and nitrogen oxides. Volcanic eruptions and wildfires, for example, release these gases into the atmosphere, although their contribution to acid rain is relatively minor compared to human activities.
- Long-Distance Transport: Pollutants emitted in one location can be carried by the wind over long distances before being deposited as acid rain. As a result, even regions far away from the original emission sources can be affected by acid rain.
The primary contributors to acid rain are sulfur dioxide and nitrogen oxides. Once these pollutants are released into the atmosphere, they can be transported over great distances before being washed out of the air through precipitation, resulting in acid rain in affected areas. To address the issue of acid rain, efforts focus on reducing emissions of sulfur dioxide and nitrogen oxides through the implementation of various environmental regulations and clean energy initiatives.
The Effects of Acid Rain
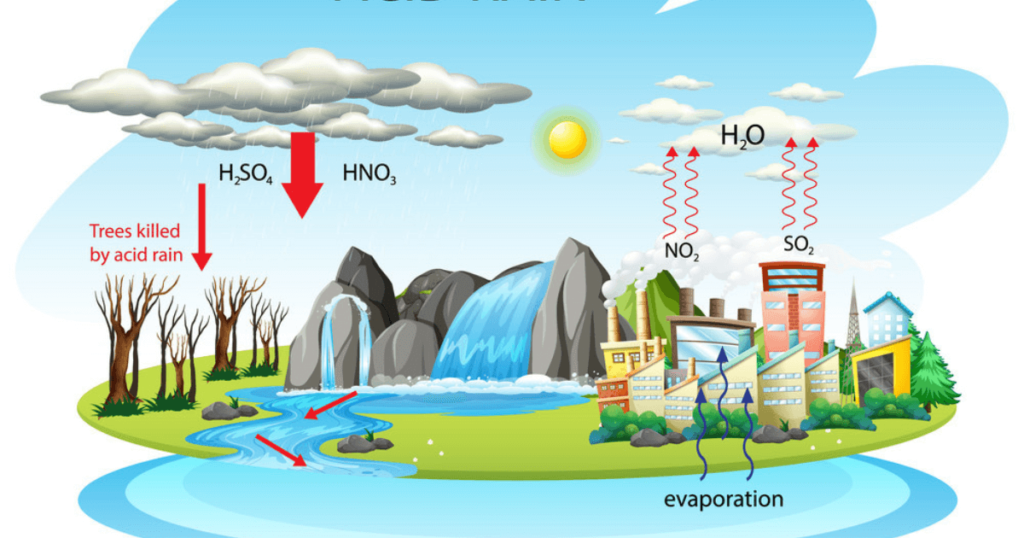
The effects of acid rain can be far-reaching and have serious consequences for the environment, ecosystems, human health, and infrastructure. Some of the key effects of acid rain include:
- Damage to Aquatic Ecosystems: Acid rain can lead to the acidification of lakes, rivers, and streams. When acid rain falls into these water bodies, it lowers their pH levels, making them more acidic. This increased acidity can be harmful to aquatic life, including fish, insects, and other organisms. It disrupts the balance of the ecosystem and may lead to the decline or loss of certain species.
- Soil Degradation: Acid rain leaches important nutrients such as calcium, magnesium, and potassium from the soil. This depletion of nutrients affects the growth and health of plants and trees. In areas with chronic acid rain, the soil may become infertile, hindering agriculture and natural vegetation.
- Damage to Vegetation: The acidic components of acid rain can directly damage vegetation. The acid can erode the protective waxy layer on leaves and needles, making them more susceptible to diseases, frost, and other environmental stressors. Acid rain can also affect the root systems of plants, further compromising their health.
- Corrosion of Buildings and Infrastructure: Acid rain reacts with materials such as limestone, marble, concrete, and metals in buildings, monuments, and infrastructure. Over time, this can cause corrosion and degradation, leading to significant damage to historical structures and modern buildings alike.
- Impact on Human Health: While direct exposure to acid rain does not pose significant health risks, the pollutants that cause acid rain (sulfur dioxide and nitrogen oxides) can contribute to air pollution. Breathing in these pollutants can irritate the respiratory system, aggravate asthma and other respiratory conditions, and make individuals more susceptible to respiratory infections.
- Damage to Monuments and Cultural Heritage: Acid rain can corrode and erode historical monuments, sculptures, and other cultural heritage sites made from materials susceptible to acid reactions, leading to irreversible damage.
- Transboundary Effects: Acid rain does not respect international borders. Pollutants released in one country can be carried by wind currents and cause acid rain in neighbouring or even distant regions, impacting ecosystems and communities beyond the source of emissions.
- Economic Costs: The effects of acid rain on agriculture, forestry, infrastructure, and public health can result in substantial economic costs, including repair and restoration expenses and losses in agricultural productivity.
To mitigate the effects of acid rain, it is essential to reduce the emission of sulfur dioxide and nitrogen oxides through strict regulations, use cleaner and renewable energy sources, and promote sustainable practices in various industries. International cooperation is also crucial to address transboundary air pollution and tackle the global issue of acid rain effectively.
Know about Bhujangasana (Cobra Pose) its benefits and precautions
Key solutions to mitigate and prevent Rain:

Addressing the issue of acid rain requires a combination of regulatory actions, technological advancements, and individual efforts. Here are some key solutions to mitigate and prevent acid rain:
- Emission Reduction: a. Implement Environmental Regulations: Enforce strict regulations on emissions of sulfur dioxide (SO2) and nitrogen oxides (NOx) from power plants, industries, and transportation sectors. Set emission limits and promote the use of cleaner technologies. b. Cap-and-Trade Programs: Establish cap-and-trade systems where industries are assigned emission allowances, and they can buy or sell permits based on their emission levels. This incentivizes companies to reduce emissions and rewards those that exceed their reduction targets.
- Use of Clean Energy: a. Transition to Renewable Energy: Promote the use of renewable energy sources, such as wind, solar, hydroelectric, and geothermal power, to reduce dependence on fossil fuels and lower emissions. b. Energy Efficiency: Encourage energy efficiency measures in industries, buildings, and transportation to decrease energy consumption and emissions.
- Flue Gas Desulfurization (FGD): Install flue gas desulfurization systems, commonly known as scrubbers, in industrial facilities and power plants. Scrubbers remove sulfur dioxide from flue gas before it is released into the atmosphere, significantly reducing emissions.
- Low-NOx Technologies: Implement low-NOx technologies in vehicles and industrial processes to minimize nitrogen oxide emissions.
- International Cooperation: Work collaboratively at the national and international levels to address transboundary air pollution. Share knowledge, technologies, and best practices to collectively combat acid rain.
- Public Awareness and Education: Increase public awareness about the causes and effects of acid rain. Educate communities about individual actions they can take to reduce their carbon footprint and contribute to cleaner air.
- Sustainable Land Management: Promote sustainable land management practices to minimize soil erosion and nutrient loss, which can help counteract the effects of acid rain on vegetation.
- Research and Monitoring: Invest in research and monitoring programs to understand the extent of acid rain’s impact on ecosystems, water bodies, and human health. This information can help guide effective policy decisions and targeted interventions.
- Acid Rain-Resistant Materials: Utilize acid rain-resistant materials in construction and infrastructure projects, especially in areas prone to acid rain, to minimize corrosion and degradation.
- Global Climate Action: Address the broader issue of climate change, as reducing greenhouse gas emissions can indirectly contribute to lowering the levels of acid rain-causing pollutants.
By adopting these solutions and implementing comprehensive strategies, we can make significant progress in reducing acid rain’s harmful effects and protecting the environment, human health, and critical infrastructure.








[…] “Understanding Acid Rain: Causes, Effects, and Sustainable Solutions” […]
[…] “Understanding Acid Rain: Causes, Effects, and Sustainable Solutions” […]